Germany continues to lead in integrating data infrastructure and molecular diagnostics into its cancer care ecosystem. With robust regulatory governance, active cross-border collaborations, and well-established Next-Generation Sequencing (NGS) capabilities, the country sets a strong example. Nevertheless, gaps in routine data sharing, reimbursement for NGS, and system-wide standardisation persist, requiring strategic policy attention.
_______________________________________________________________________________
Quick access
Explore Germany’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake

Germany benefits from a mature governance structure, with BfARM overseeing data related to insurance, service providers, and administrative records. The country is also highly active in cross-border and cross-disciplinary collaborations, though routine data sharing among centres remains limited.
Key strengths of the German data ecosystem:
- Oversight of data sharing through a designated controlling body.
- Strong data security governance (internal and external guidelines) and established data linking with EHRs.
- Active participation in cross-border collaborations for research and data exchange.
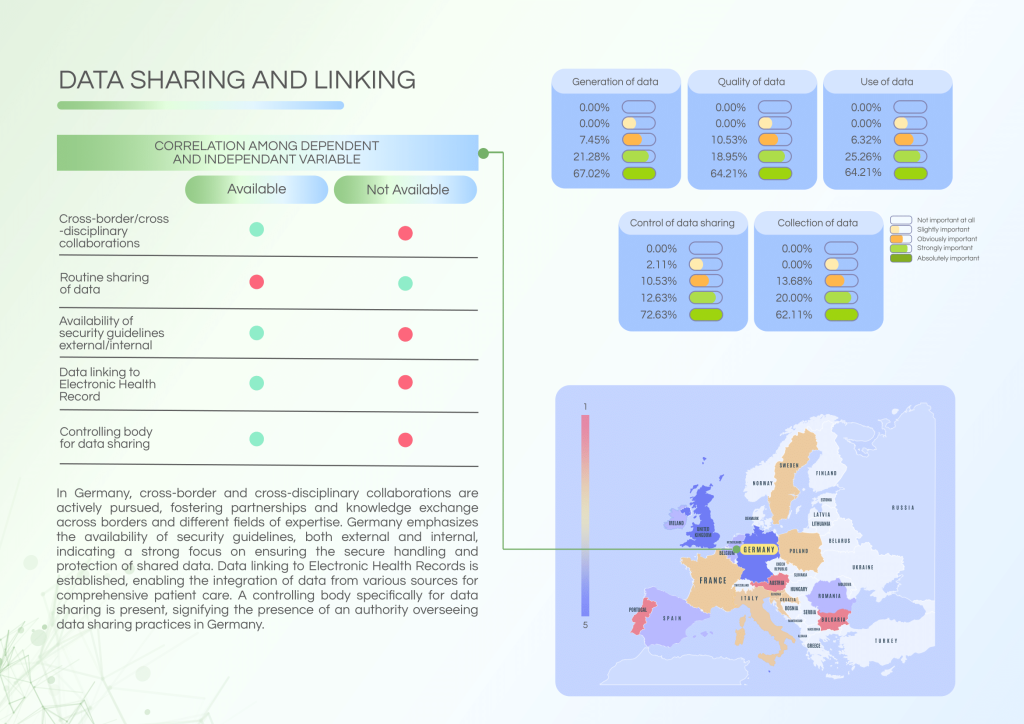
Persistent challenges:
- Routine data sharing practices across institutions are not fully established.
- Variability in patient communication post-NGS testing among centres.
- Continued need to enhance standardisation of internal protocols.
Policy recommendations:
- Foster routine data sharing agreements among cancer centres.
- Develop and implement harmonised patient communication standards for NGS testing.
- Continue strengthening internal governance mechanisms for data use and sharing.
Revolutionising Molecular Diagnostics
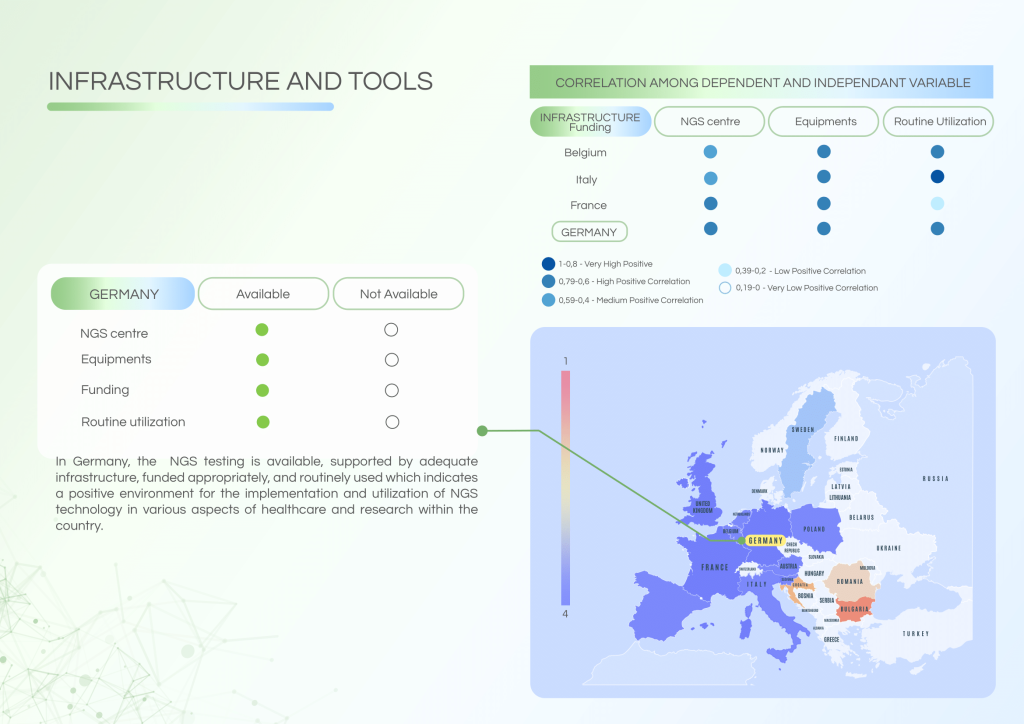
Germany has successfully embedded NGS in routine clinical workflows, supported by adequate infrastructure, a skilled workforce, and established Molecular Tumour Boards (MTBs). Despite this, reimbursement for NGS procedures remains limited, and data-sharing practices could be improved for broader benefit.
System strengths include:
- Routine NGS testing across certified centres.
- Well-functioning MTBs for comprehensive diagnosis and treatment planning.
- A highly trained workforce with extensive awareness of NGS applications.
- Availability of structured educational programmes to support continuous professional development.
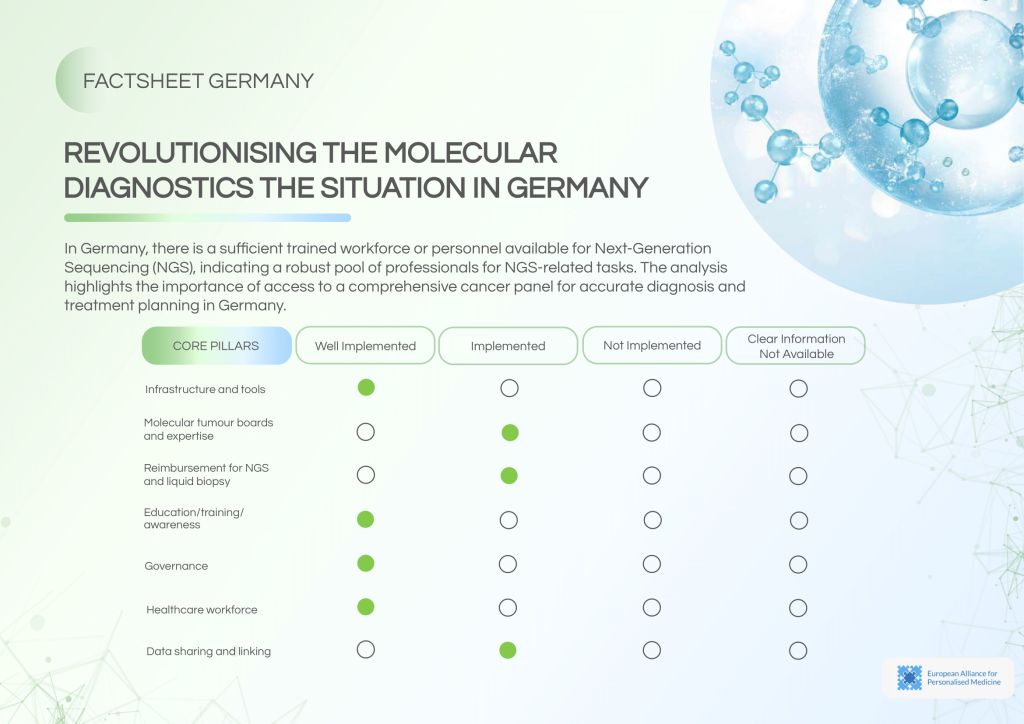
Ongoing challenges:
- Reimbursement for NGS is not universally available, posing a barrier to access.
- Data sharing beyond research contexts remains limited to ad-hoc agreements.
- Despite ISO accreditation availability, variability in internal guidelines persists.
Recommended actions:
- Simplify and broaden reimbursement pathways for NGS and liquid biopsy.
- Enhance inter-institutional data-sharing frameworks beyond clinical studies.
- Strengthen ISO certification adoption and harmonise internal clinical protocols.
- Continue investing in education and workforce development to maintain high standards.