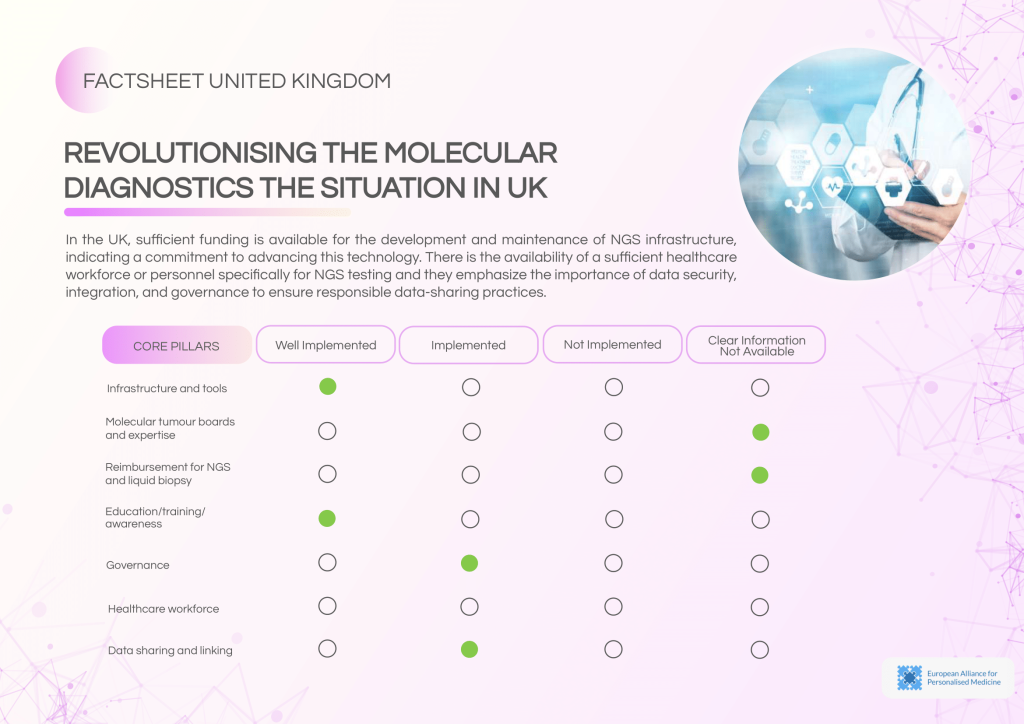
The United Kingdom (UK) has a mature infrastructure for health data management and genomic integration, with multiple umbrella bodies regulating data sharing and interoperability. Routine NGS implementation and well-established clinical guidelines are in place. However, challenges remain in streamlining cross-border collaborations, optimising molecular diagnostic governance, and ensuring broader clinical adoption of NGS through sustainable reimbursement frameworks. Addressing these gaps will be vital to advancing the UK’s leadership in precision oncology and personalised cancer care.
_______________________________________________________________________________
Quick access
Explore the UK’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake

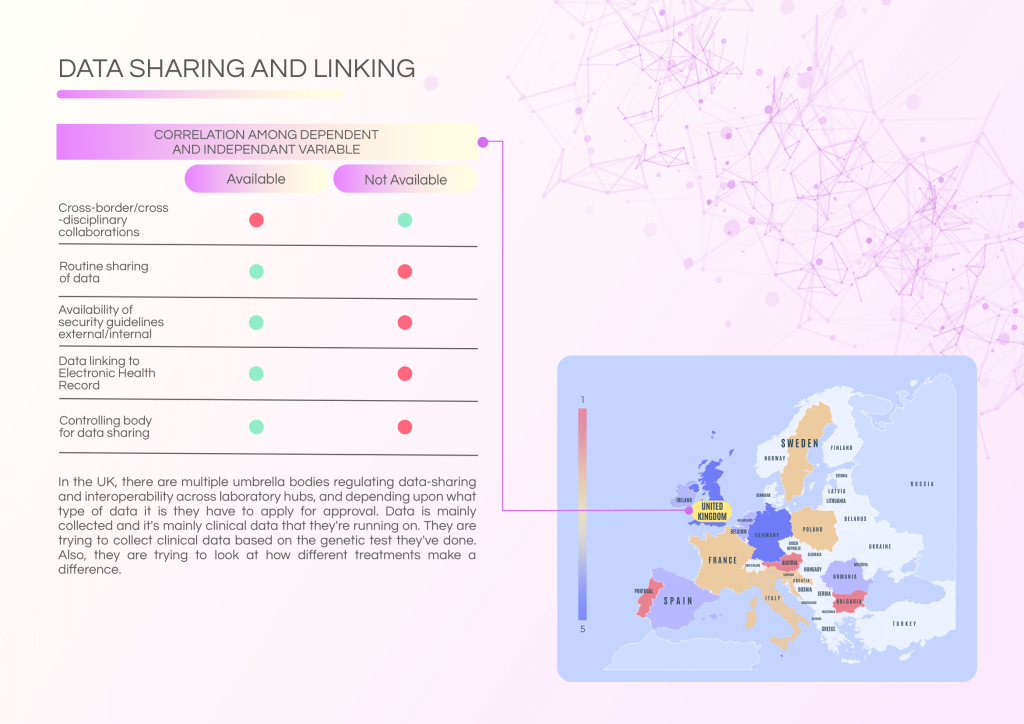
The UK has a well-established data governance ecosystem with multiple regulatory bodies ensuring data sharing and interoperability across laboratory hubs. Data linking to Electronic Health Records (EHRs) is routinely practiced, supported by robust security guidelines.
Strengths of the UK’s data ecosystem:
- Routine data sharing and established governance structures.
- Data linking with EHRs for clinical application and research.
- Strong emphasis on data security and integration.
- Controlling body oversight ensures compliance with regulations.
Challenges and areas for development:
- Complex and fragmented approval processes for data sharing.
- Limited cross-border and cross-disciplinary collaborations.
- Opportunities to expand public sector data linkage for research.
Policy recommendations:
- Simplify the legal framework for data sharing in research.
- Enhance collaborations among research institutions and healthcare stakeholders.
- Promote harmonised guidelines for genomic data use and patient communication.
- Data linking to EHRs is limited beyond individual patient care contexts.
- Cross-border collaborations and multi-institutional data exchange are minimal.
- Routine sharing of genomic data across cancer centres is inconsistent.
Revolutionising Molecular Diagnostics
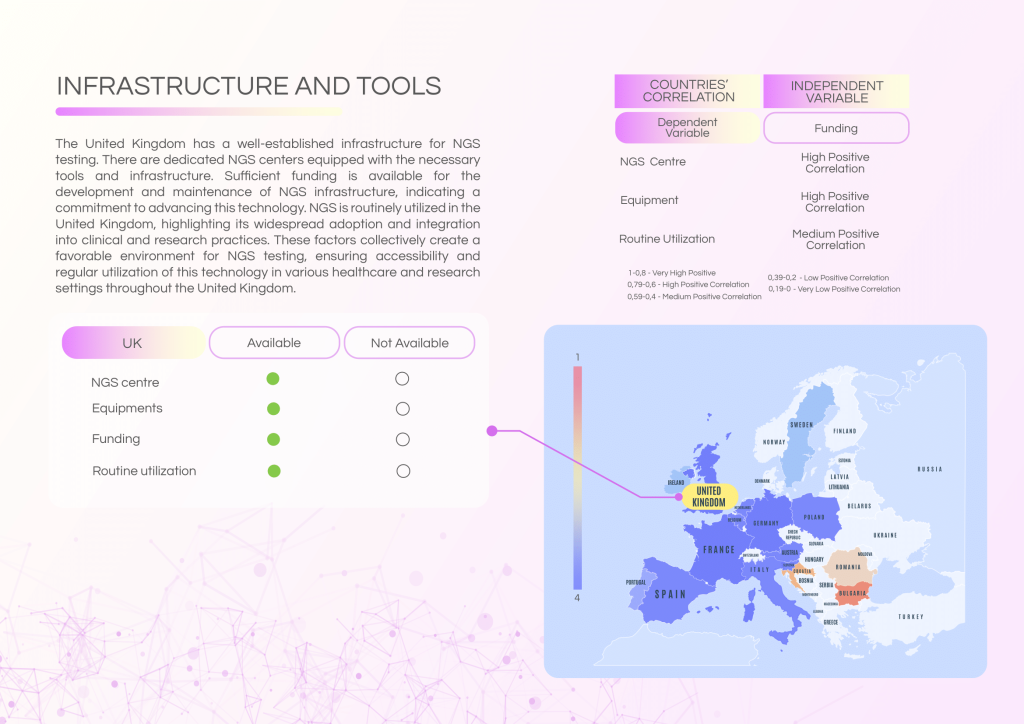
The UK benefits from well-funded NGS infrastructure and a sufficient healthcare workforce trained in NGS technologies. However, governance, reimbursement, and formalised molecular tumour board (MTB) structures require further attention to ensure equitable and widespread adoption.
Strengths of the molecular diagnostics landscape:
- Routine NGS utilisation across clinical and research settings.
- Adequate funding for NGS infrastructure and equipment.
- High awareness and educational programmes on NGS applications.
- Strong workforce capacity with trained personnel.
Ongoing challenges:
- Absence of dedicated MTBs for molecular diagnostics decision-making.
- Low patient volume for MTB discussions.
- Lack of ISO accreditation and limited external quality assessment practices.
- Reimbursement status for NGS and liquid biopsy is not clearly defined.
Recommended actions:
- Expand efforts to integrate molecular diagnostics into standard care protocols.
- Establish a national MTB framework for structured clinical decision-making.
- Develop clear reimbursement pathways for NGS and liquid biopsy.
- Promote ISO certification and external quality assurance.