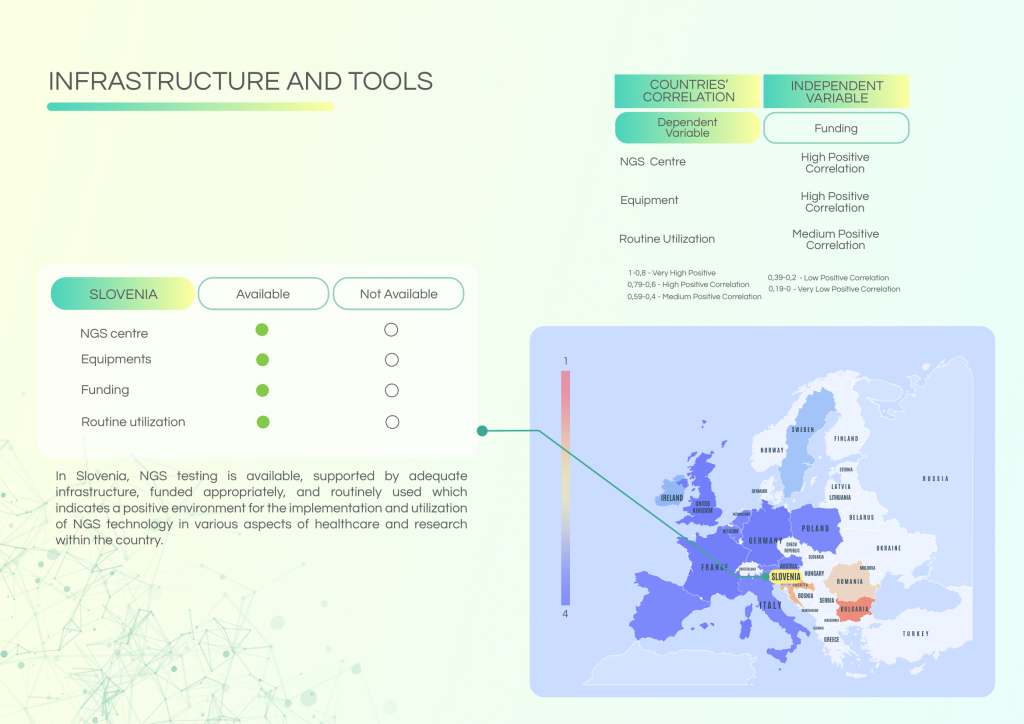
Slovenia has made steady progress in integrating genomic data and applying Next-Generation Sequencing (NGS) in clinical practice. Key advancements include data linkage with Electronic Health Records and coordinated national data platforms. However, challenges persist in cross-border collaborations, workforce capacity, and the availability of education and governance mechanisms. Addressing these gaps will be essential to fully translating Slovenia’s existing infrastructure into practical, patient-centred personalised cancer care.
_______________________________________________________________________________
Quick access
Explore Slovenia’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake
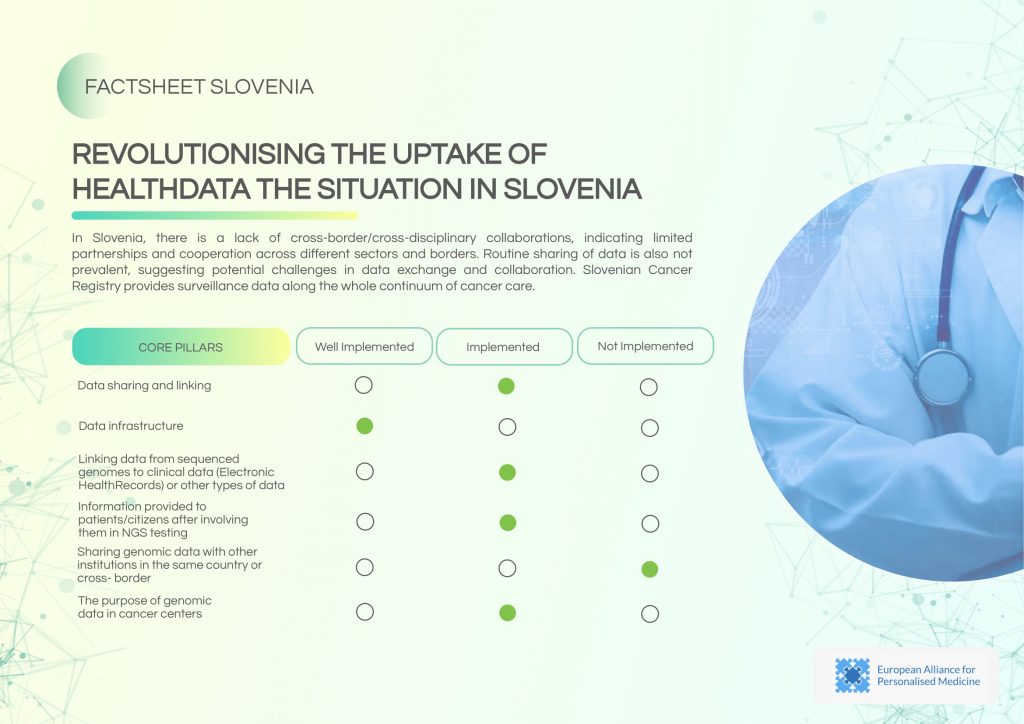
Slovenia demonstrates a high level of confidence in its data infrastructure, with established linkages to Electronic Health Records (EHRs) and a dedicated controlling body for data sharing. However, cross-border collaborations and routine data exchange remain limited.
Key strengths of Slovenia’s data landscape:
- Functional data linking between genomics and EHR systems.
- Active national-level data coordination through the Central Registry of Patient Data.
- Availability of external and internal security guidelines.
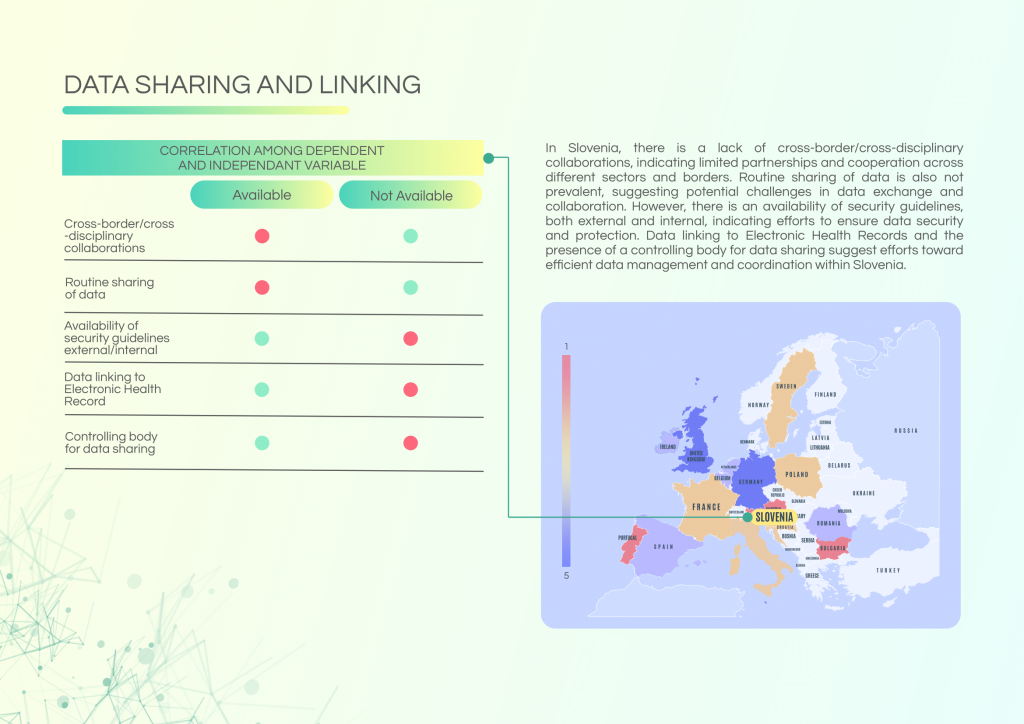
Challenges to address:
- Limited cross-border and cross-sector collaborations.
- Routine data sharing is not consistently practiced.
- Need for enhanced patient information frameworks related to NGS testing.
Policy recommendations:
- Foster international collaborations for data sharing and innovation.
- Develop standardised patient communication protocols for NGS testing.
- Enhance trust and transparency in genomic data use among stakeholders.
Revolutionising Molecular Diagnostics
NGS testing is available and supported by adequate infrastructure in Slovenia, with recurring Molecular Tumour Board (MTB) meetings. However, workforce shortages, low awareness, and insufficient education initiatives hinder the widespread adoption of molecular diagnostics.
Current strengths:
- Operational NGS centres with routine utilisation.
- Established MTBs with weekly consultation meetings.
- Reimbursement pathways exist for NGS and liquid biopsy.
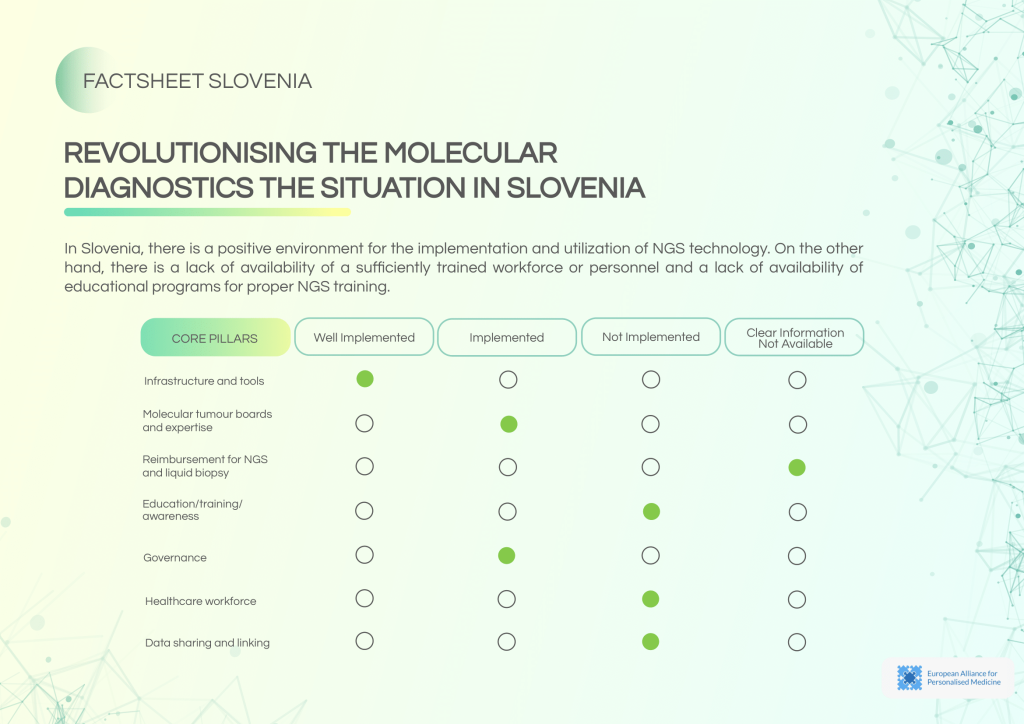
Persistent barriers:
- Lack of trained personnel to support NGS expansion.
- No dedicated educational programmes for NGS awareness and training.
- ISO accreditation is not widely adopted.
- External quality assessments are present but need broader application.
Recommended actions:
- Expand workforce development programmes in genomics and bioinformatics.
- Implement nationwide educational initiatives to raise awareness.
- Support ISO certification adoption across NGS service providers.
- Secure sustainable funding to maintain reimbursement mechanisms.