Poland is gradually advancing its personalised cancer care capabilities, with active cross-border collaborations and data linkage practices. However, challenges in governance, routine data sharing, and diagnostic infrastructure limit the widespread implementation of Next-Generation Sequencing (NGS) in clinical practice. Bridging the gap between research potential and practical application remains a critical priority.
_______________________________________________________________________________
Quick access
Explore Poland’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake

Poland demonstrates strong participation in cross-border and cross-disciplinary collaborations. While data linking to Electronic Health Records (EHRs) is practiced, routine data sharing remains limited and lacks centralised governance.
Strengths of Poland’s data landscape:
- Established data linkage with EHRs in Comprehensive Cancer Centres.
- Participation in cross-border collaborations for research and innovation.
- Security guidelines for data handling are in place.
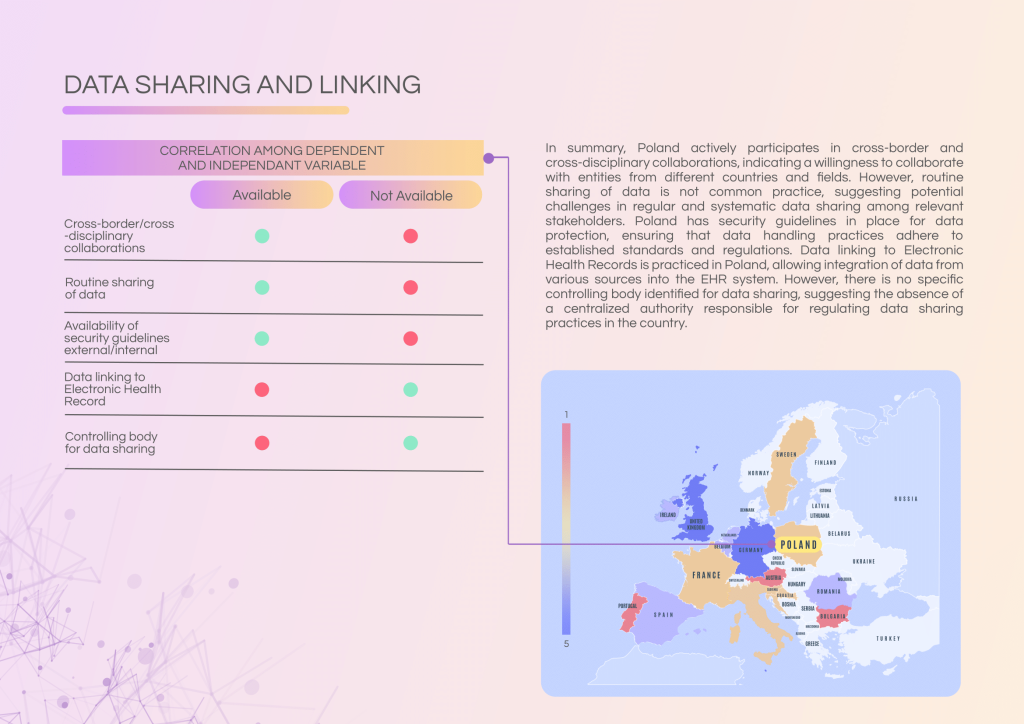
Key gaps and challenges:
- Routine data sharing practices are not common among centres.
- No central controlling body oversees data sharing at a national level.
- Low confidence in data infrastructure for NGS implementation.
- National Cancer Registry data are underutilised for quality assessment.
Policy recommendations:
- Establish a dedicated data governance authority.
- Develop national standards for routine data sharing and integration.
- Improve utilisation of registry data for monitoring cancer care quality.
- Enhance public and patient engagement in genomic data initiatives.
Revolutionising Molecular Diagnostics
Poland’s NGS adoption remains at an early stage, with limited infrastructure and clinical application. Despite having equipment and trained personnel, the country faces challenges in scaling NGS to routine use due to governance gaps, lack of reimbursement pathways, and workforce limitations.
Current system strengths:
- Basic NGS infrastructure and equipment available in certain centres.
- Cross-border collaborations fostering knowledge exchange.
- Recognised need for national strategy in genomic data governance.

Main barriers:
- No national reimbursement for NGS or liquid biopsy procedures.
- Absence of Molecular Tumour Boards (MTBs) for structured clinical discussions.
- Low awareness and training availability for NGS applications.
- Limited workforce capacity in bioinformatics and molecular diagnostics.
Priority actions:
- Establish a national MTB framework to integrate NGS into clinical workflows.
- Develop reimbursement pathways for NGS and liquid biopsy.
- Expand training programmes to address the workforce gap.
- Implement ISO certification and quality assurance for NGS testing.


