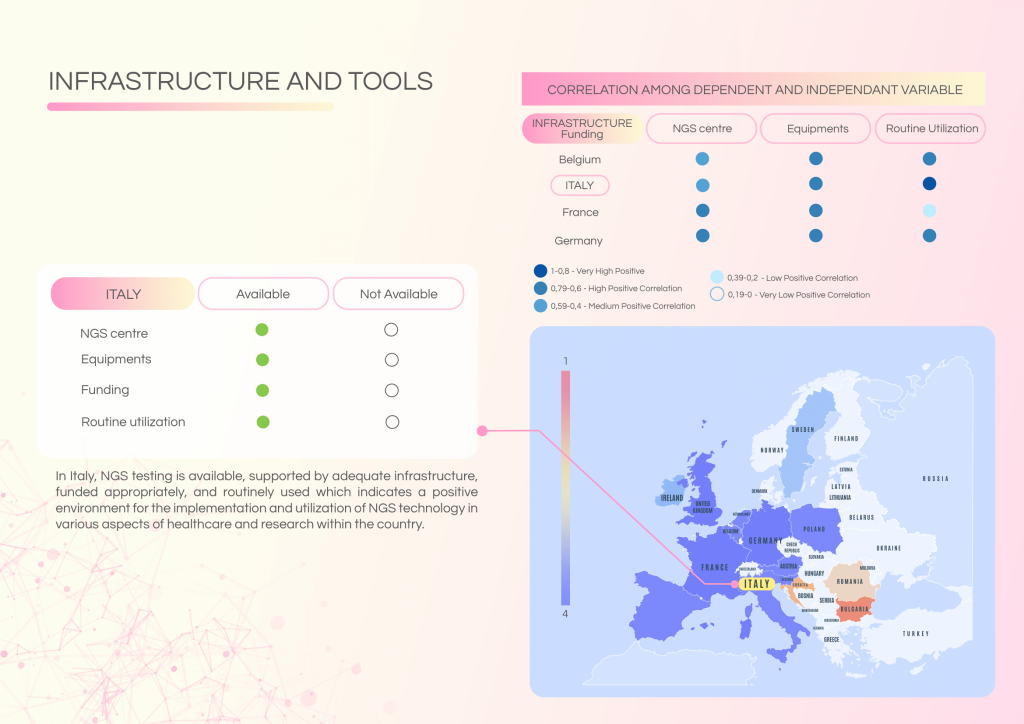
Italy has made significant strides in personalised oncology, with robust data infrastructures and active cross-border collaborations. The integration of NGS data with clinical records is established, yet challenges persist in governance, workforce capacity, and the practical application of molecular diagnostics in everyday care. Bridging the gap between research and clinical practice remains a key priority.
_______________________________________________________________________________
Quick access
Explore Italy’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake
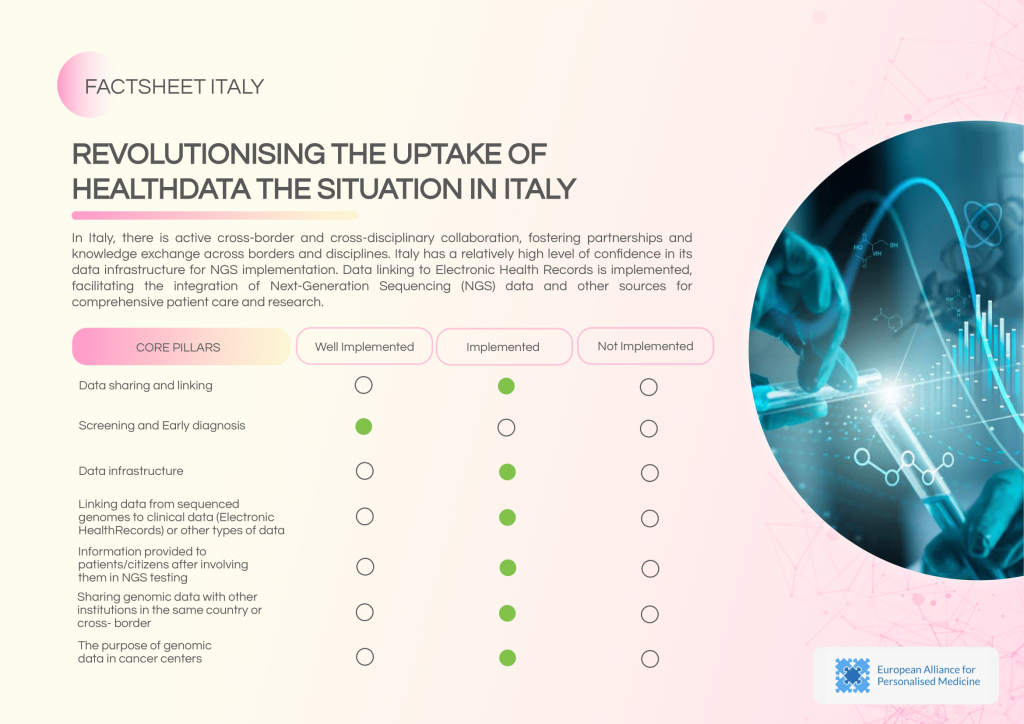
Italy demonstrates a high level of confidence in its data infrastructure for NGS implementation, with established data linking to Electronic Health Records (EHRs) and active cross-border collaborations. However, governance gaps and inconsistencies in data security frameworks remain challenges.
Strengths of Italy’s health data landscape:
- Well-established data linking between genomic data and EHRs.
- Active cross-border and cross-disciplinary collaborations.
- Routine data sharing for research and innovation purposes.
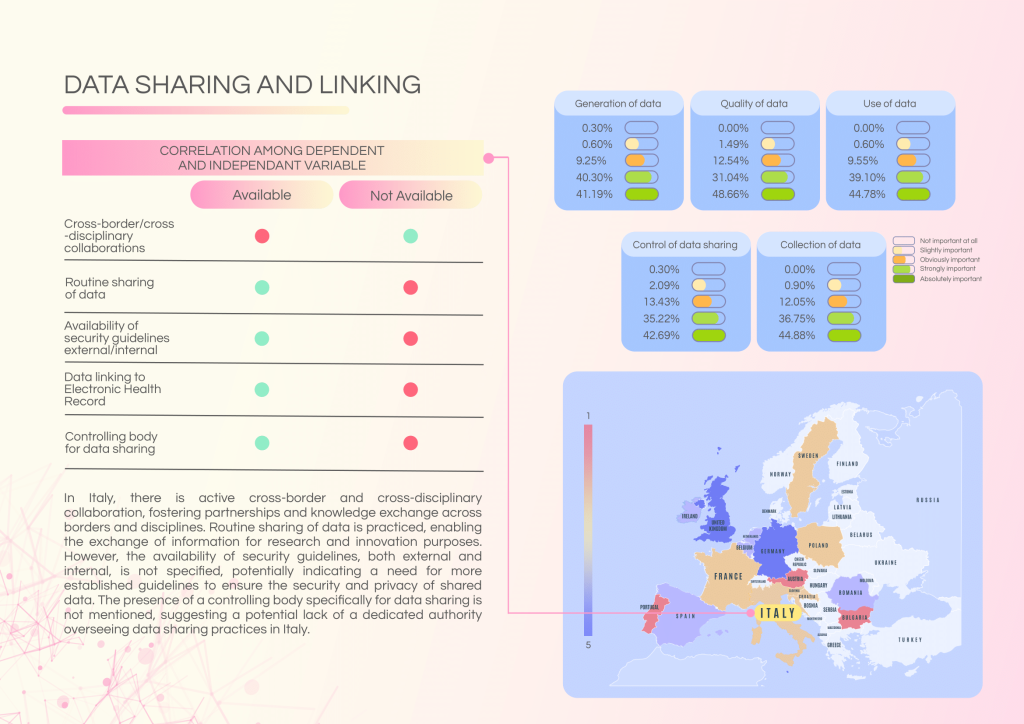
Challenges and gaps:
- Absence of a central controlling body specifically for data sharing.
- Unspecified security guidelines (internal and external) for data handling.
- Discrepancies in patient communication standards post-NGS testing.
Policy recommendations:
- Establish a national governance authority for data sharing and security oversight.
- Develop standardised guidelines for genomic data protection and patient communication.
- Address regional disparities in cancer screening and data usage.
Revolutionising Molecular Diagnostics
Italy has developed a solid foundation for molecular diagnostics with accessible NGS centres, appropriate funding, and ISO accreditation pathways. Despite this, a significant gap persists between research-oriented NGS use and its integration into routine clinical practice.
System strengths include:
- Functional NGS infrastructure and funding for implementation.
- Availability of ISO accreditation for labs performing NGS.
- Recurring Molecular Tumour Board (MTB) meetings, though at varied levels.
- Reimbursement available for NGS, with dedicated funding for liquid biopsy in lung cancer.
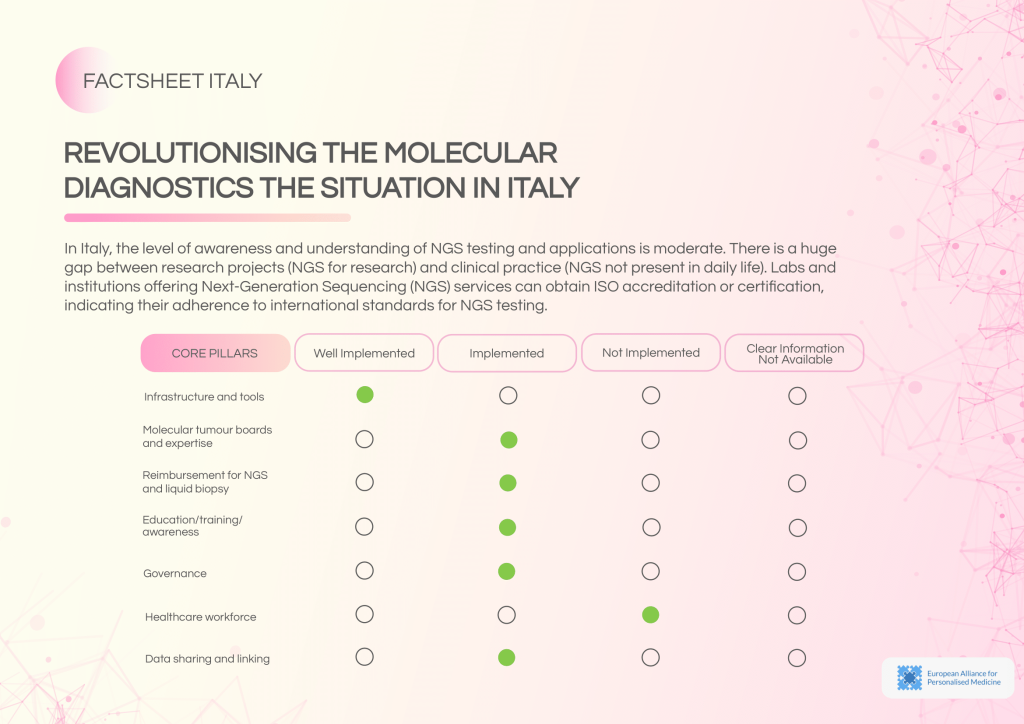
Barriers and gaps:
- No established MTB structure for regular molecular tumour consultations.
- Lack of reimbursement pathways for NGS and liquid biopsy.
- Absence of ISO accreditation and limited external quality assessment.
- Moderate awareness of NGS applications, with no dedicated national awareness initiatives.
Recommended actions:
- Develop and fund a national MTB network to standardise molecular consultations.
- Implement reimbursement schemes for NGS and liquid biopsy.
- Support adoption of ISO accreditation and formal quality assurance measures.
- Expand training and awareness initiatives to enhance clinical adoption.