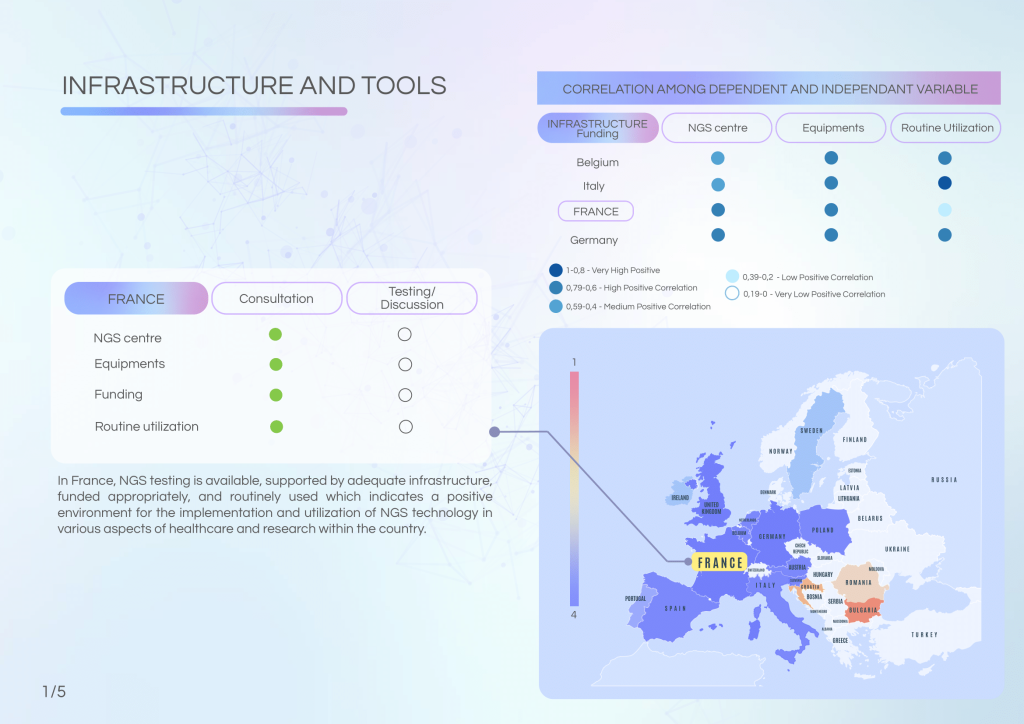
France is a frontrunner in integrating data infrastructure and molecular diagnostics into cancer care. With a robust Health Data Hub, active Molecular Tumour Boards (MTBs), and routine Next-Generation Sequencing (NGS) usage, the country demonstrates a strong commitment to precision oncology. Nonetheless, challenges remain in areas such as reimbursement clarity, workforce capacity, and standardisation of data-sharing practices.
_______________________________________________________________________________
Quick access
Explore France’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake
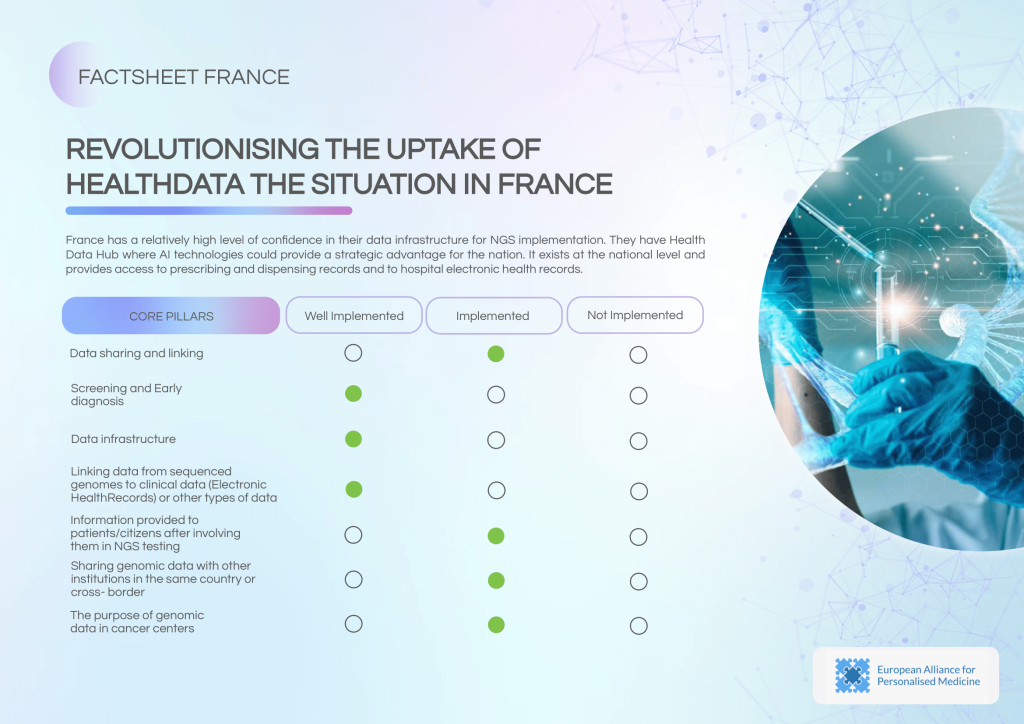
France benefits from a well-established Health Data Hub that integrates prescribing data, dispensing records, and hospital EHRs, providing a strong foundation for data-driven oncology care. However, the country faces limitations in cross-border data sharing and still needs to enhance collaborations and standard protocols.
Strengths of the French health data ecosystem:
- High confidence in data infrastructure for NGS implementation.
- Established security guidelines (internal and external) with oversight by a controlling body.
- Functional data linking to Electronic Health Records (EHRs).
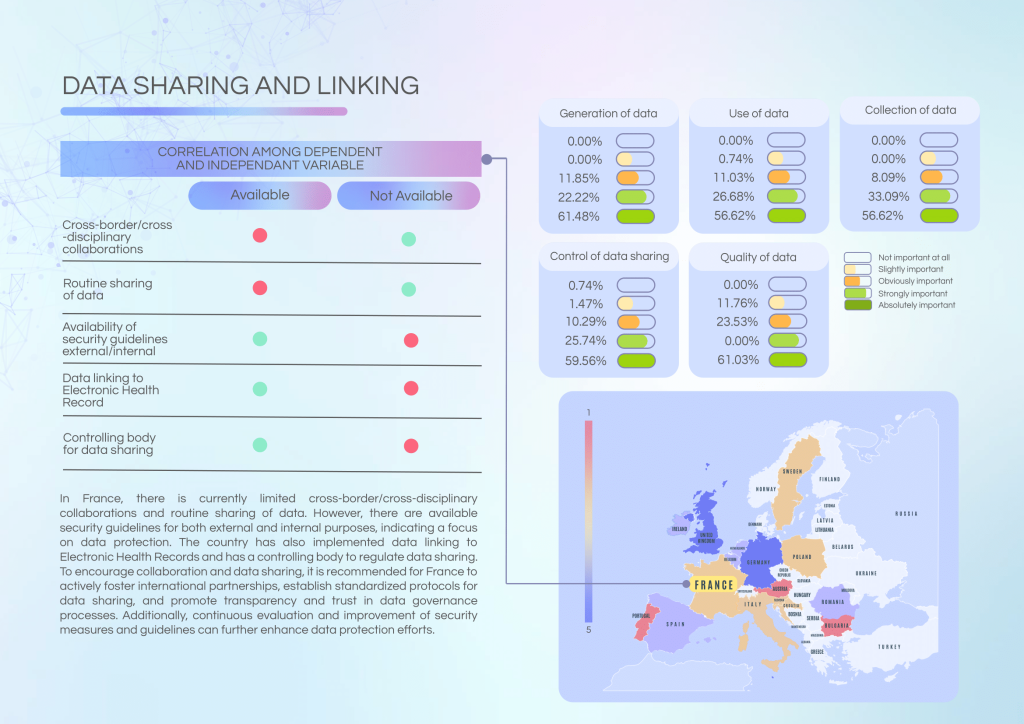
Key challenges and gaps:
- Limited routine cross-border data sharing and collaboration.
- Absence of ISO accreditation across many labs and lack of internal guidelines.
- Variability in patient information delivery post-NGS testing across centres.
Policy recommendations:
- Foster international collaborations and harmonised data-sharing standards.
- Strengthen internal governance protocols within laboratories and cancer centres.
- Standardise patient communication and reporting practices linked to NGS testing outcomes.
Revolutionising Molecular Diagnostics
France has successfully integrated NGS into clinical and research settings, supported by adequate infrastructure and funding. The presence of specialised MTBs and recurring consultation meetings highlights the country’s structured approach. However, reimbursement complexity, workforce shortages, and governance gaps persist.
Key strengths of the molecular diagnostics landscape:
- Broad availability and routine use of NGS testing.
- Well-established Molecular Tumour Boards (MTBs) for diagnosis and treatment planning.
- Ongoing education and training programmes to enhance professional capacity.
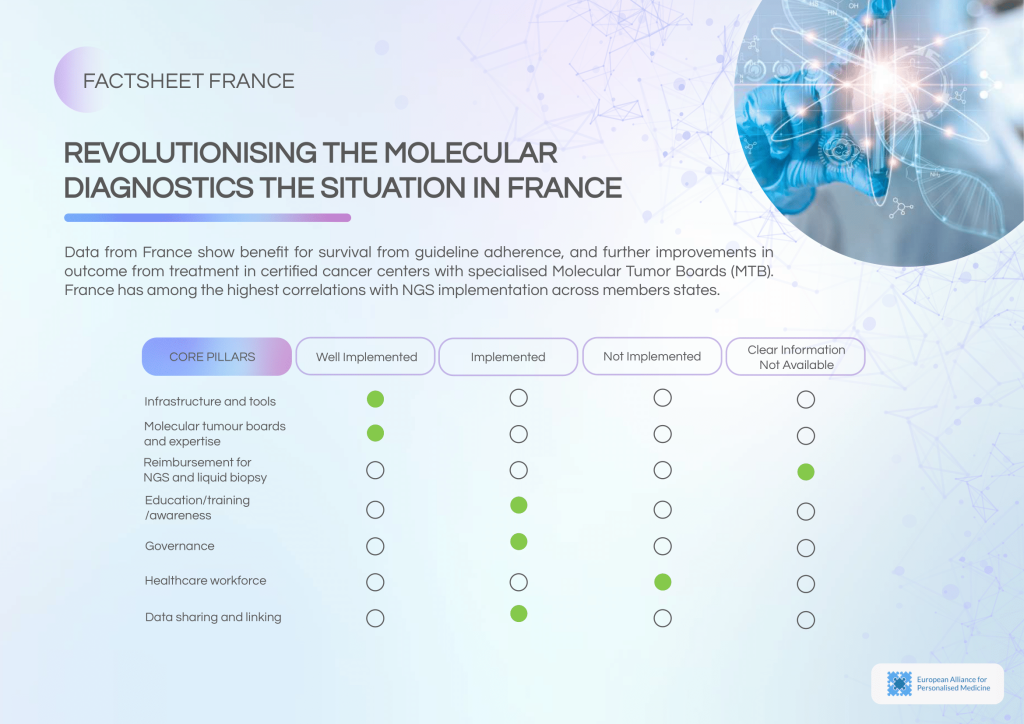
Persistent barriers:
- A complex and fragmented reimbursement system impedes wider adoption.
- Workforce shortages in cancer genomics hinder service delivery.
- Lack of ISO certification and absence of internal guidelines in many institutions.
- Limited cross-centre data-sharing in routine practice beyond clinical studies.
Recommended actions:
- Simplify and harmonise reimbursement pathways for NGS and liquid biopsy.
- Expand training initiatives to address workforce capacity challenges.
- Implement external quality assessments and move towards ISO accreditation.
- Promote data-sharing frameworks beyond clinical trials into routine care.