Ireland is progressing steadily in its efforts to integrate genomics and data-driven approaches into cancer care. While confidence in data infrastructure and the presence of NGS facilities is high, key gaps remain in governance, reimbursement, and the routine use of molecular diagnostics. Ireland’s decentralised data governance and the absence of a national MTB framework highlight areas requiring targeted policy interventions.
_______________________________________________________________________________
Quick access
Explore Ireland’s progress across two key areas of personalised cancer care. One factsheet explores the national health data ecosystem, while the other analyses the state of molecular diagnostics and NGS implementation.
Click below to access each document directly.
Revolutionising Health Data Uptake

Ireland’s national data infrastructure demonstrates high confidence for NGS implementation, but systemic challenges hinder full realisation. A lack of an integrated health information system and no centralised data-sharing authority leads to fragmentation.
Key strengths of the current landscape:
- High confidence in data infrastructure for genomics.
- Data is shared at the national level, with some cross-border exchanges.
- Strong GDPR compliance, though sometimes limiting pseudonymisation flexibility.
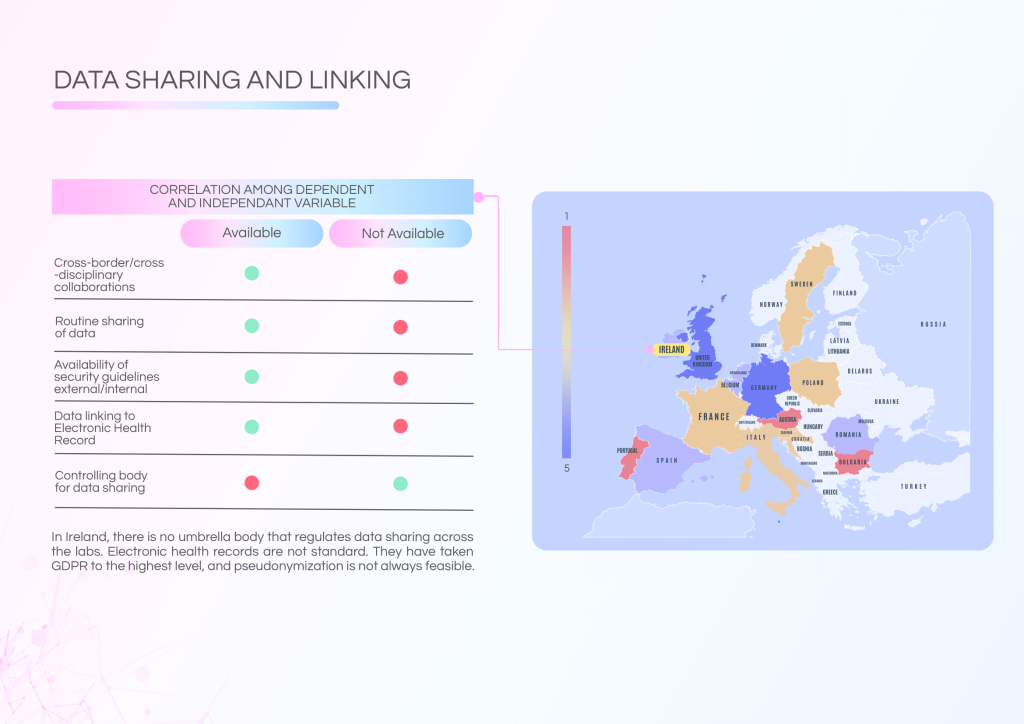
Challenges to address:
- No central body overseeing data sharing across laboratories.
- Non-standardised Electronic Health Records (EHRs) hinder genomic data linkage.
- Patient communication is inconsistent despite the availability of summarised NGS reports.
Policy recommendations:
- Strengthen patient communication frameworks in line with Europe’s Beating Cancer Plan.
- Establish a national coordinating body for data sharing governance.
- Standardise EHR systems and genomic data integration protocols.
Revolutionising Molecular Diagnostics
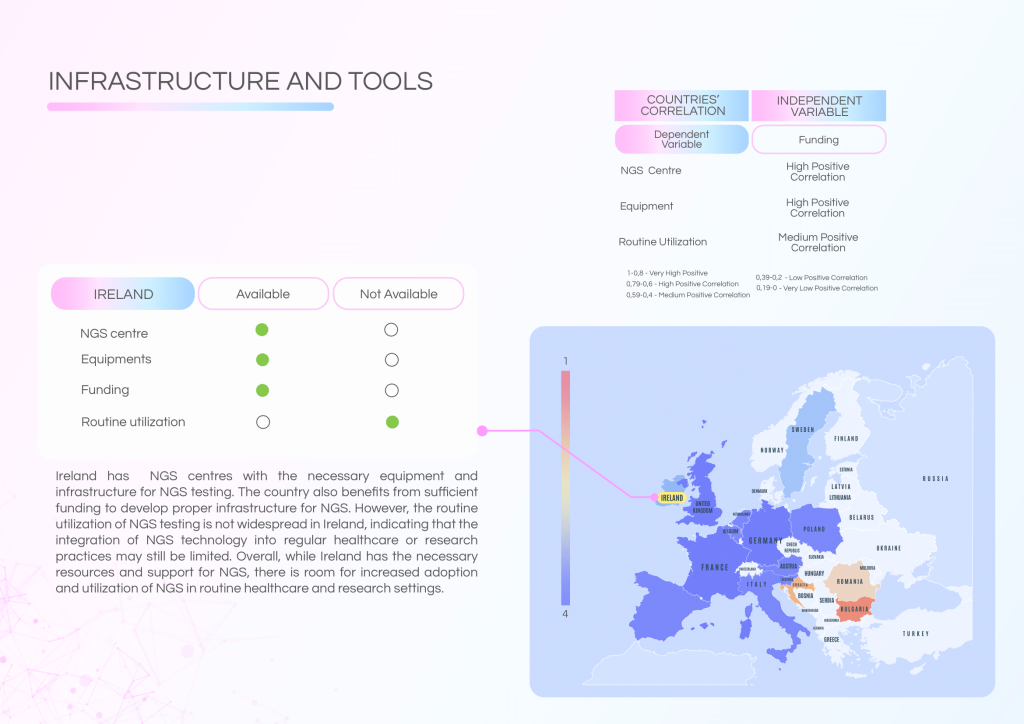
Ireland has developed solid infrastructure for NGS testing and benefits from a skilled workforce. However, routine clinical utilisation of NGS remains limited, and critical gaps in governance, reimbursement, and Molecular Tumour Board (MTB) frameworks persist.
Current strengths include:
- Strong technical expertise in clinical, laboratory, and bioinformatics domains.
- Established NGS centres with adequate equipment and funding.
- Educational programmes providing proper training for NGS procedures.

Barriers and gaps:
- No established MTB structure for regular molecular tumour consultations.
- Lack of reimbursement pathways for NGS and liquid biopsy.
- Absence of ISO accreditation and limited external quality assessment.
- Moderate awareness of NGS applications, with no dedicated national awareness initiatives.
Recommended actions:
- Develop and fund a national MTB network to standardise molecular consultations.
- Implement reimbursement schemes for NGS and liquid biopsy.
- Support adoption of ISO accreditation and formal quality assurance measures.
- Expand training and awareness initiatives to enhance clinical adoption.